History
A 49 year old male presented with adenocarcinoma of the ampulla of Vater after having failed all conventional treatments.
The patient initially developed painless jaundice about 1 year previously, and was found to have a cancer of the ampulla. He sought treatment at one of the top cancer hospitals in Canada due to the rare tumour type. He was treated like a case of pancreatic cancer, and he had a Whipple procedure performed. Regional lymph nodes were found to be involved at the time of surgery, so he was given chemotherapy (5-FU) and radiation. After about 10 months, the patient developed multiple liver metastases, so treatment was changed to gemcitabine. This was not effective, so Tarceva was tried. When it was determined that Tarceva was also ineffective, the patient was told that nothing more could be done. He then came to our clinic.
There was a history of thyroid surgery (no thyroid cancer), but the patient was otherwise healthy. He was taking Dilaudid 0.5mg about 4-5 times a day, L-thyroxine and Tarceva. He was also taking some natural supplements including Milk Thistle and Deep Immune. He was losing weight, having loose floating bowel movements after every meal, feeling short of breath, and having daily fevers and chills. He was also experiencing a significant amount of right upper quadrant abdominal pain.
Findings
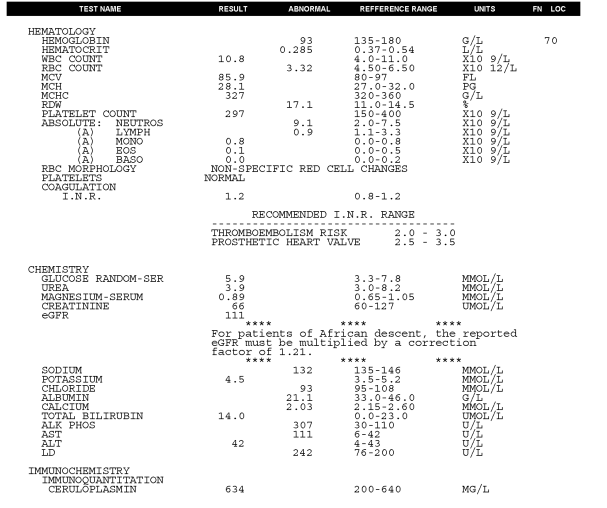
Body weight was 70.4kg. Vital signs were normal, there was no fever. Oxygen saturation was 95% at rest. Air entry to the right lower lung was reduced. The abdomen was mildly distended, the liver was enlarged and tender and there was ascites present. There was allodynia in the region of his Whipple incision. There was significant bilateral ankle swelling. There were multiple abnormalities seen on the initial blood tests (see Fig 6A).
Fig. 6A – pre-treatment blood tests
Treatment
We offered a comprehensive approach to properly manage the pain, improve nutritional status and treat the cancer. A naturopathic consultation was also arranged to provide optimization of the natural medications.
The patient was counseled on correct use of pain medications, and Lyrica was added to treat the neuropathic component of the pain. Pancreatic enzymes were started and increased until the bowel movements were formed. Tarceva was stopped. We decided to treat the cancer with a combination of DCA + TM.
DCA 500mg t.i.d. (=21mg/kg/day, 2 weeks on / 1 week off) + TM 40mg t.i.d. were started. We gave the patient routine supplementation with R alpha lipoic acid 150mg t.i.d. and benfotiamine 80mg b.i.d. to help prevent DCA side effects. Within 1 week, the patient’s fevers disappeared. There was also a reduction in ascites, a reduction in pain and the ankle swelling nearly resolved. Significant improvements began to appear on the blood tests after only 1 week. Over the next 4 weeks, many blood parameters showed steady improvements (Fig 6B – 6E).
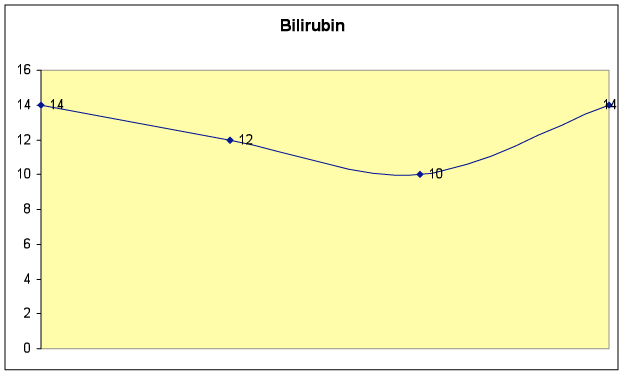
Fig. 6B – Bilirubin level dropped initially, and remained in the normal range
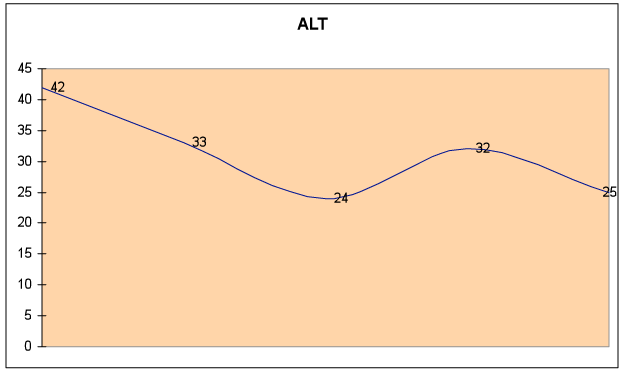
Fig. 6C – ALT remained in the normal range (<43), but improved by 43%
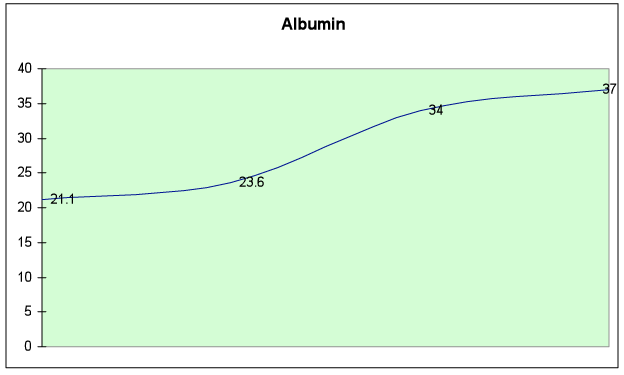
Fig. 6D – Albumin level returned to normal (>35)
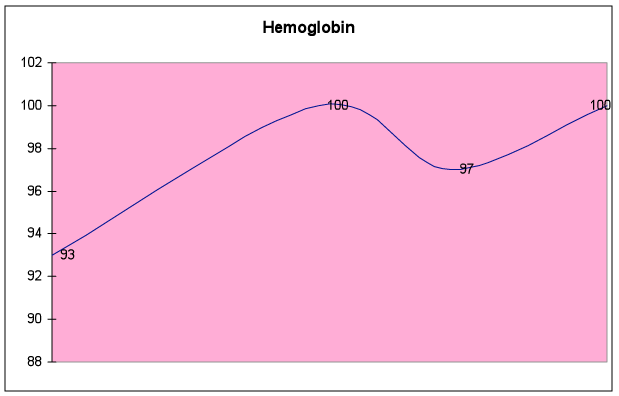
Fig. 6E – Hemoglobin improved by 8%
The patient was able to eat better and weight loss had stopped. Ankle swelling was gone. At the same time, there was a mild increase in liver pain.
At this point the target range for copper deficiency was nearly achieved, but the blood cell counts all started to drop (due to rapid copper-lowering). Because of pre-existing anemia, hemoglobin dropped to 83 (normal 135) so TM had to be stopped temporarily. Eprex 40000U s.c. weekly injections were initiated to help correct the underlying cancer-related anemia with the hope that TM could be quickly re-started.
After 4 weeks of therapy, there was a sudden marked deterioration in liver function and symptoms. Despite increasing the dose of DCA up to 57mg/kg/day (3 days on and 7 days off), no improvement was seen. A number of other treatment options were being considered when the patient required admission to hospital due to increased ascites and difficulty breathing. Unfortunately due to hospital policies, all off-label cancer treatments had to be stopped during admission. Thus TM could not be re-started, and other treatment options could not be explored.
Comments
This case illustrates a very dramatic, but short-lived response to DCA of a very aggressive and rare cancer that was resistant to chemotherapy in a patient with no conventional treatment options available. He benefited from improved quality of life (and probable life extension) of about 1 month with no serious side effects.
This case also illustrates how DCA can have a role in the treatment of rare cancers in the absence of clinical trial data. The reason DCA may be used in such cases is that the drug is very safe when compared to chemotherapy or other “standard” therapies that are toxic, immune-suppressing, or have life-threatening side effects.
It is unfortunate to see short-lasting responses in some patients. It is possible that a subgroup of the cancer cells were sensitive to DCA and died rapidly, leaving the resistant cells behind to eventually grow back and make the patient ill again. We have new data from ChemoFit testing which suggests this may be the case (see the ChemoFit section of this website for information about this test).
It is possible that TM when combined with DCA may have been more effective for this patient. Unfortunately, hospitals often prohibit off-label therapies (like TM in this case), despite the fact that gentle off-label therapies can often help patients significantly.