History
A 42 year old male requested telemedicine consultation for treatment of glioblastoma. He developed unremitting headaches was diagnosed with a grade 2 right parietal oligodendroglioma about 2 years prior to consulting us. This was treated initially with surgical debulking (90% of the tumour was removed).
Due to re-growth 1 year after surgery, he was treated with temozolamide chemotherapy, with good initial results. After 7 cycles of temozolamide, the tumour began to re-grow.
The patient was then treated with chemo-radiation (combination temozolamide + radiation). He improved initially after this treatment, but was not given any good options for further treatment in the event of new growth. At that time, an MRI showed the tumour way 6.5cm in its maximum dimension (anterior-posterior). There was a notation in the report of possible increase in contrast enhanced material within tumour suggesting early re-growth after chemo-radiation.
He then consulted us about novel treatment approaches.
The patient had a history of high blood pressure, anxiety/depression, possible sleep apnea, and had smoked for 25 years (he quit after the cancer diagnosis).
Medications at the time were oxazepam, valium, imovane, methylprednisolone 4mg q.i.d. (tapering after radiation), and acetaminophen 1000mg t.i.d. – q.i.d. as needed.
The patient had some symptoms from the tumour including left-sided numbness, weight loss, low energy, sweats/chills, and intermittent pain. Body weight was 111kg.
Treatment
We recommended comprehensive treatment including a combination of DCA + TM, dietary changes, management of depression and DVT prevention.
The DCA dose was 2500mg per day divided into 2 doses (=23mg/kg/day) on cycle of 2 weeks on / 1 week off. We supplemented this with R-alpha lipoic acid 150mg t.i.d. and benfotiamine (vitamin B1) 80mg b.i.d. The TM dose was 40mg t.i.d. which was to be reduced after 4 weeks to 20mg t.i.d. – q.i.d. depending on CBC and ceruloplasmin results. We handed over the management of the TM dose to the patient’s own doctor.
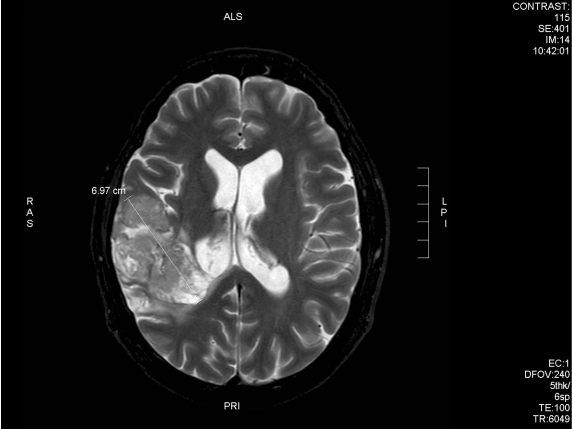
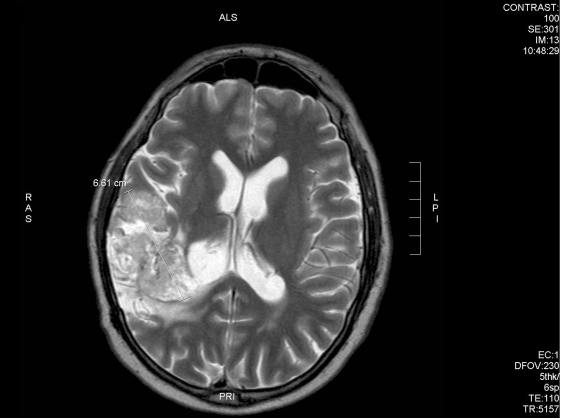
After 6 weeks of treatment, the patient noted improvement in symptoms and he obtained a new MRI scan which showed a reduction of the tumour (Fig 9A and 9B).
Fig. 9A – 3 months following chemo-radiation (prior to DCA treatment start) the tumour is 6.95cm in its maximum dimension (anterior-posterior)
Fig. 9B – After 6 weeks of DCA treatment, tumour reduced by 4mm
The patient continues to tolerate DCA + TM very well after a total of 4 months of treatment. His symptoms were stable, and he has reported no significant side effects from these medications.
Comments
This case demonstrates the safety and significant benefits which are possible with gentle non-chemo treatments like DCA and TM. This patient has an excellent quality of life and is not suffering from debilitating medication side effects.
We believe that the combination of DCA+TM can clearly improve quality of life for patients with very aggressive tumours like glioblastoma, and most likely will also improve survival. It will take randomized clinical trials to prove that with greater certainty, but due to the non-proprietary status of drugs like DCA and TM, such trials will likely never take place.